THINKING SPACE NATURE
HOW TO TURN SUNLIGHT INTO HYDROGEN
“Water is the coal of the future. The energy of tomorrow will be provided by water that is broken down into its elements by electric current. The elements of water – hydrogen and oxygen – thus decomposed, will satisfy the energy demand on earth for the indefinite future.”
What the writer Jules Verne anticipated in his novel “The Mysterious Island” [1] in 1870, is nowadays known as the hydrogen economy. Researchers all over the world are currently working on the further development of this promising technology. In the future, it could also push forward the energy turnaround in regions with high sunshine duration.
HYDROGEN: CLEAN AND POWERFUL
The basic idea behind the new technology: solar energy is stored in hydrogen. If this hydrogen is used as a fuel, it burns to form harmless water vapour and provides a great deal of energy – more than is contained in the same amount of gasoline or in a battery of similar weight.
NATURE AS A ROLE MODEL: PHOTOSYNTHESIS IN PLANTS
Plants show us how it’s done. By means of photosynthesis, they store solar energy in carbohydrates. The pigment chlorophyll, which is found in the leaves of plants, plays an important role in this process: it absorbs sunlight and converts it into chemical energy. Plants use this energy to build an organic compound from water and carbon dioxide, namely glucose. The sugar makes plants grow – before eventually becoming “fuel” for animals and humans in the food chain.
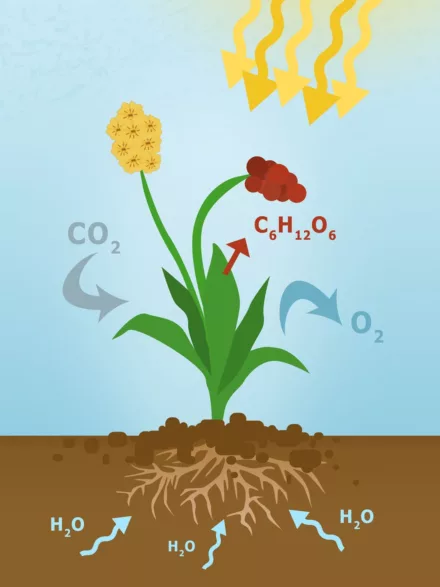
Photosynthesis takes place in the plant leaves. It is the prerequisite for the plant’s metabolism and energy conversion.
FROM SUNLIGHT TO HYDROGEN
Researchers all over the world are working to develop a technology that functions similarly to photosynthesis: with the power of the sun, an energy-rich chemical compound – namely hydrogen – is created directly in an artificial leaf. If this hydrogen is used in fuel cells such as those powering electric cars, no exhaust gases are released.
FUEL CELLS ON THE RISE?
The development of this new technology has not yet really taken off. Reasons include the costs involved – which are currently still high – and the resulting low demand for hydrogen. However, all this could soon change. According to research by the Handelsblatt [2], more and more companies worldwide are either producing hydrogen or using fuel cells to generate green electricity from it. A new path for the future – and a chance for artificial photosynthesis?
Experts are convinced that, subject to a research breakthrough, humans will be able to use sunlight more efficiently than plants. In 2011, US chemist and Harvard professor Daniel Nocera developed a leaf for artificial photosynthesis, and, in 2016, together with his colleague Pamela Silver, a low-cost liquid fuel. In Technology Review [3], Professor Nocera quotes Microsoft founder Bill Gates:
BILL GATES HAS SAID THAT TO SOLVE OUR ENERGY PROBLEMS, SOMEDAY WE NEED TO DO WHAT PHOTOSYNTHESIS DOES, AND THAT SOMEDAY WE MIGHT BE ABLE TO DO IT EVEN MORE EFFICIENTLY THAN PLANTS. THAT SOMEDAY HAS ARRIVED.
GLIMPSE INTO THE ELECTRICITY LAB: THE ELECTROLYSER
If renewable energy sources are used, hydrogen can be produced in a particularly ecofriendly manner. Direct water splitting with the help of sunlight, for instance, can be conducted by photoelectrochemical solar cells. Check out how it works at Futurium – and in our graphics:
CLIMATE-FRIENDLY ENERGY FROM HYDROGEN
Photoelectrochemical solar cells are comparable to an artificial leaf: they convert visible light into electrical energy. In the Futurium model, an LED lamp takes over the task of the sun. The light it emits sets the electrolysis in motion.
Electrolysis takes place in water: the photoelectrochemical solar cell at the bottom of the beaker uses solar energy and catalysts to split water into the two gases hydrogen and oxygen. In our model, a wedge placed above the solar cell mechanically separates the two rising gas bubbles. Electrolysers with optimised spacing usually deploy for this purpose a membrane that is impermeable to gases, but which allows ions to pass through to ensure charge-transport in the electrolyte. In our model, the ionic conductor consists of water enriched with potassium hydroxide.
If electricity is needed, hydrogen and oxygen are recombined in a fuel cell. They react with one another – thus releasing the previously stored energy – and this sets in motion the plane in the Futurium model. What you don’t see in the model: apart from energy, this process has no side product other than water. The water that is produced in the fuel cell is discharged again through small holes in the cell.